A research team from the University of Hong Kong (HKU) has deconstructed the process by which genetic information in human DNA is “read,” revealing how the wrong “reading” can lead to developmental defects and various cancers, bringing hope to the development of new anti-cancer drugs. The results have been published in the journal Science.
All types of cells in the human body have almost identical DNA sequence, known as genes. When making a particular type of cell, such as a stem cell or neuron, each cell carefully “chooses” which genes to express. Gene expression is regulated by various histone protein modifications, and errors in this process can lead to serious diseases such as cancer.
Different types of histone modifications can emit different signals to control gene expression, DNA replication, and repair. To discover how various histone modifications achieve their biological functions, we must first identify the “readers” that can recognize them.
“Readers” are a class of proteins that recognize specific histone modifications and thereby “translate” them by regulating the expression of the corresponding genes. However, many “readers” are still unidentified, thus limiting the scientific understanding of gene regulation.
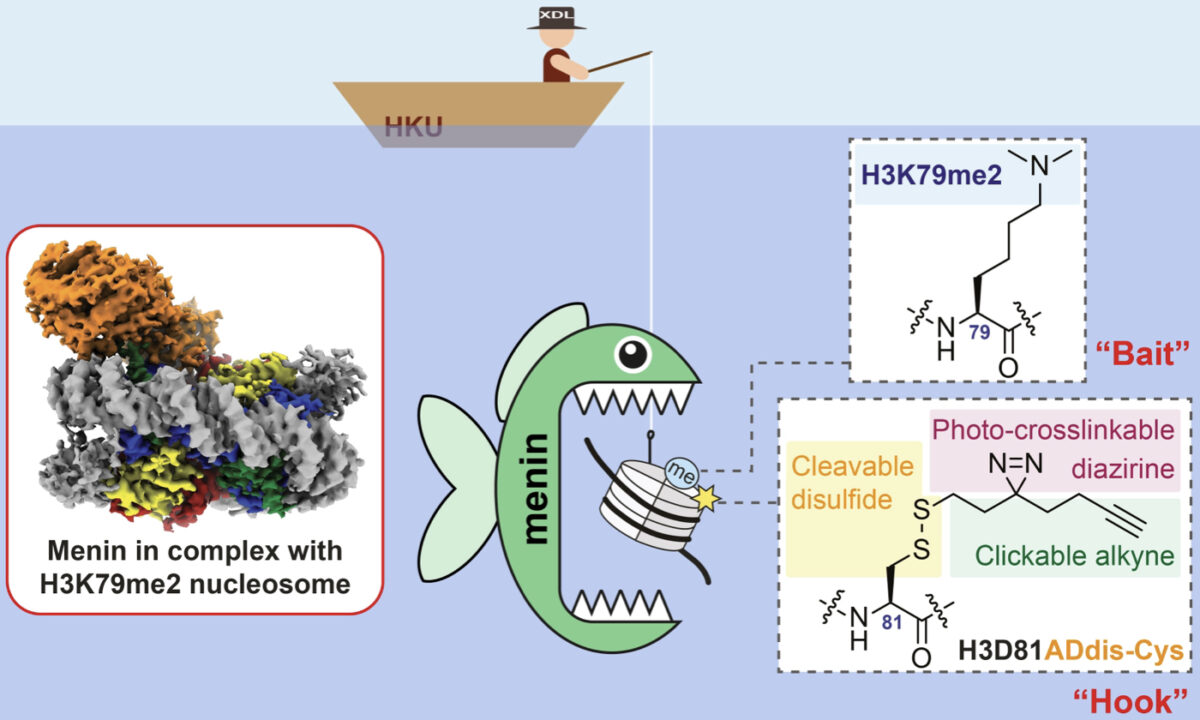
Professor Xiang Li’s research team at HKU’s department of chemistry has been dedicated to identifying the “readers” of various histone modifications. One approach is to use histone-modified peptides, small segments of histones, as “bait,” and “hooks” equipped with photo-activated chemical groups to “fish” for these “readers” by irradiating UV light.
Li’s team has made a breakthrough in the study of the methylation modification of histone H3 lysine 79 (H3K79me2). An H3K79me2 deficiency in mammalian embryos can lead to a variety of developmental abnormalities, including growth disorders, cardiac dilation, and even death. In many cancers, including childhood leukemia, H3K79me2 is substantially higher than normal and even appears in locations where it should not.
“To capture the H3K79me2 ‘readers,’ we must upgrade our ‘bait’ and ‘hook,'” said Li. It took them more than five years to develop the new tool, using a chemically synthesized intact nucleosome with H3K79me2 modification as the “bait” and installing the new “hook” on the synthesized nucleosome, to successfully identify a menin protein as the “reader” of H3K79me2.
To understand how menin “reads” the H3K79me2 marker, the team used cryo-electron microscopy to present the molecular details of their interactions, which are millions of times smaller than the resolution limit of the human eye. “Unravelling the details of how menin binds H3K79 methylation is key to designing new drugs to treat cancers associated with [dysregulated] H3K79me2,” said Li.