

"This overhaul of the clinical trials legislation will ... help to streamline approvals by removing granular and duplicative regulatory requirements," Marc Bailey, Chief Science and Innovation Officer at the Medicines and Healthcare products Regulatory Agency (MHRA) said.
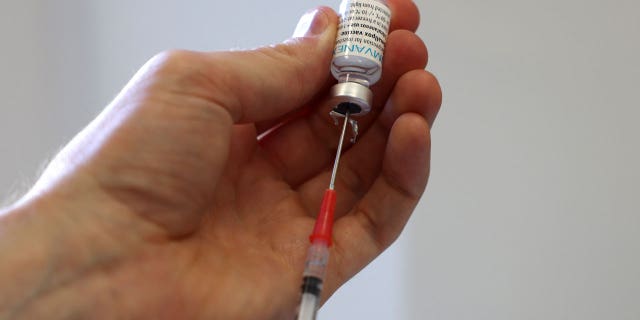
A medical professional prepares a dose of the monkeypox vaccine on July 23, 2022, in London, England. Britain is set to overhaul clinical trial regulations for a faster and easier track for approval. (Photo by Hollie Adams/Getty Images)
An application review will need to be completed within 30 days, while a maximum of 10 days will be given for a decision to be granted, the regulator said.
The measures, which the MHRA said would be the biggest overhaul in two decades, will introduce a legal mandate to register the trial in a World Health Organization public register, and will require publishing a summary of results within 12 months after the end of the trial.
